Some interesting facts about the Periodic Table
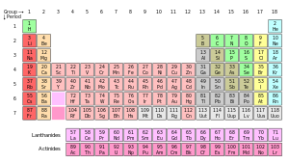
In virtually every chemistry classroom on the planet, there is a chart known as the Periodic Table of Elements. At first glance, it looks like a mere series of boxes, with letters and numbers in them, arranged according to some kind of code not immediately clear to the observer. The boxes would form a rectangle, 18 across and 7 down, but there are gaps in the rectangle along the top. To further complicate matters, two rows of boxes are shown along the bottom, separated from one another and from the rest of the table. One needs to really understand and appreciate the information to realize that it is one of the most efficient yet simple means ever designed for representing the basic building blocks of matter.
How was the table created?
Did you know that the Periodic Table of elements was created as early as 1869?
By the time Mendeleev constructed his periodic table in 1869, there were 63 known elements. He assembled the elements in increasing order of theiratomic masses on a chart. As Mendeleev observed, every eighth element on the chart exhibits similar characteristics, and thus, he established columns whereby element number x was placed above element number x + 8—for instance, helium (2) above neon (10). The patterns he observed were so regular that for any “hole” in his table, he predicted that an element to fill that space would be discovered.
Dmitri Mendeleev
Indeed, Mendeleev was so confident in the basic soundness of his Table that in some instances, he changed the figures for the atomic mass of certain elements because he was convinced they belonged elsewhere on the table. Later discoveries of isotopes, which in some cases affected the average atomic mass considerably, confirmed his suppositions. Likewise the undiscovered elements he named “eka-aluminum,” “eka-boron,” and “eka-silicon” were later identified as gallium, scandium, and germanium, respectively.
Although it has undergone some modifications, the original Table created by Dmitri Mendeleev holds even today in its form and structure.
Introductory Concepts
The Periodic Table is a method of systematic classification of chemical elements so that their study becomes easier.
Each box in the table represents an element by its chemical symbol, along with its atomic number and its average atomic mass in atomic mass units.
The rows of the periodic table of elements are called periods. The elements of a period are characterized by the fact that they have the same number of electron shells; the number of electrons in these shells, which equals the element’s atomic number, increases from left to right within each period. In each period the lighter metals appear on the left, the heavier metals in the center, and the nonmetals on the right. Elements on the borderline between metals and nonmetals are called metalloids.
The vertical columns are known as groups. In the new IUPAC system, columns are numbered with Arabic numerals from 1 to 18. These group numbers correspond to the number of s, p, and d orbital electrons added since the last noble gas element (in column 18). This is in keeping with current interpretations of the periodic law which holds that the elements in a group have similar configurations of the outermost electron shells of their atoms. Since most chemical properties result from outer electron interactions, this tends to explain why elements in the same group exhibit similar physical and chemical properties.
Group 1 (with one valence electron) and Group 2 (with two valence electrons) are called the alkali metals and the alkaline-earth metals, respectively. Two series of elements branch off from Group 3, which contains the transition elements, or transition metals; elements 57 to 71 are called the lanthanide series, or rare earths, and elements 89 to 103 are called the actinide series, or radioactive rare earths
The nonmetals in Group 17 (with seven valence electrons) are called the halogens. The elements grouped in the final column (Group 18) have no valence electrons and are called the inert gases, or noble gases, because they react chemically only with extreme difficulty.
Blocks
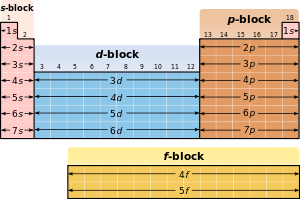
Because of the importance of the outermost electron shell, the different regions of the periodic table are sometimes referred to as blocks, named according to the sub shell in which the last electron resides. The s-block comprises the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p-block comprises the last six groups which are groups 13 to 18 in IUPAC and contains, among others, all of the metalloids The d-block comprises groups 3 to 12 in IUPAC and contains all of the transition metals. The f-block, usually offset below the rest of the periodic table, comprises the lanthanides and actinides.
Various blocks of the Periodic Table
Isotopes and atomic mass
You will observe that some of the elements have fractional atomic masses. But all atoms contain protons and neutrons (each having mass of approximately 1 amu) in whole numbers. Puzzling? Read on.
While working with English chemist Frederick Soddy, Rutherford discovered that when an atom emitted certain types of particles, its atomic mass changed. Rutherford and Soddy named these atoms of differing mass isotopes, though at that point—because the neutron had yet to be discovered—they did not know exactly what change had caused the change in mass. Certain types of isotopes, Soddy and Rutherford went on to conclude, had a tendency to decay by emitting particles or gamma rays, moving (sometimes over a great period of time) toward stabilization. In the process, these radioactive isotopes changed into other isotopes of the same element—and sometimes even to isotopes of other elements.
Soddy concluded that atomic mass, as measured by Berzelius, was actually an average of the atomic masses of all the isotopes of the element. This explained a problem with Mendeleev’s periodic table, in which there seemed to be irregularities in the increase of atomic mass from element to element.
For example, Chlorine has two stable and naturally occuring isotopes: 35Cl (with a relative abundance of 75%) and 37Cl (with a relative abundance of 25%), and hence its atomic mass, which is the average of the atomic mass of its two isotopes, is,
m= (0.75*35 +0.25*37) = 35.5
Hence, the average atomic mass of various isotopes of an element is given in the periodic table. This explains why some of the atomic masses are fractions even though subatomic particles such as protons and neutrons are present in whole numbers.
Trends in the periodic table
The chemical properties of an element depend on its valence electrons which are most readily available for participation in chemical reactions. Hence those with the same number of outermost electrons are generally grouped together.
Many properties of elements follow particular trends in the Periodic Table. Some of them are explained below.
Trends in the Periodic Table
Atomic and ionic radii
The radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period.
Reason: As the atomic number increases along each row of the periodic table, the additional electrons go into the same outermost shell; whose radius gradually contracts, due to the increasing nuclear charge. In a noble gas, the outermost shell is completely filled; therefore, the additional electron of next alkali metal will go into the next outer shell, accounting for the sudden increase in the atomic radius.
Ionization energy and reactivity
Ionization energy is the energy required to remove an electron from an isolated atom. Since a reaction involves sharing or transfer of electrons, it is also a measure of the reactivity of an atom.
Moving left to right within a period or upward within a group, the first ionization energy generally increases.
Reason: Moving left to right within a period or upward within a group, the first ionization energy generally increases.
Electronegativity
Electronegativity may be defined as the tendency of an atom to pull the shared pair of electrons involved in bonding towards itself. It is a measure of the ability of an atom to gain electrons easily.
In general, electronegativity increases on passing from left to right along a period, and decreases on descending a group. Hence, fluorine is the mist electronegative element in the Periodic Table.
Reason: Since atomic radii decrease across the period, the electrostatic attraction between the nucleus and the incoming electron increases. This makes the electronegativity higher. Similarly, as more shells are added down the group, the distance from the nucleus increases and the attraction reduces. Hence the decrase in electronegativity down the group.
From this article,I hope I have succeeded in making you realize the importance of the Periodic Table for a chemist, and the wealth of information it provides him with.